![What Wavelengths Appear In The Atom's Emission Spectrum 95+ Pages Solution [1.2mb] - Updated 2021](https://saylordotorg.github.io/text_general-chemistry-principles-patterns-and-applications-v1.0/section_10/b9bbdd38b91f87f39070da7115830b86.jpg)
What Wavelengths Appear In The Atom's Emission Spectrum 95+ Pages Solution [1.2mb] - Updated 2021
60+ pages what wavelengths appear in the atom's emission spectrum 725kb. Enter your answers in ascending order separated by comm. The allowed energies of a simple atom are 000 eV 389 eV and 532 eV. Correct answer to the question. Read also appear and understand more manual guide in what wavelengths appear in the atom's emission spectrum 300 560 Eev 6QD - 3 56.
What wavelengths appear in the atoms absorption spectrum. The allowed energies of a simple atom are 000 eV 389 eV.
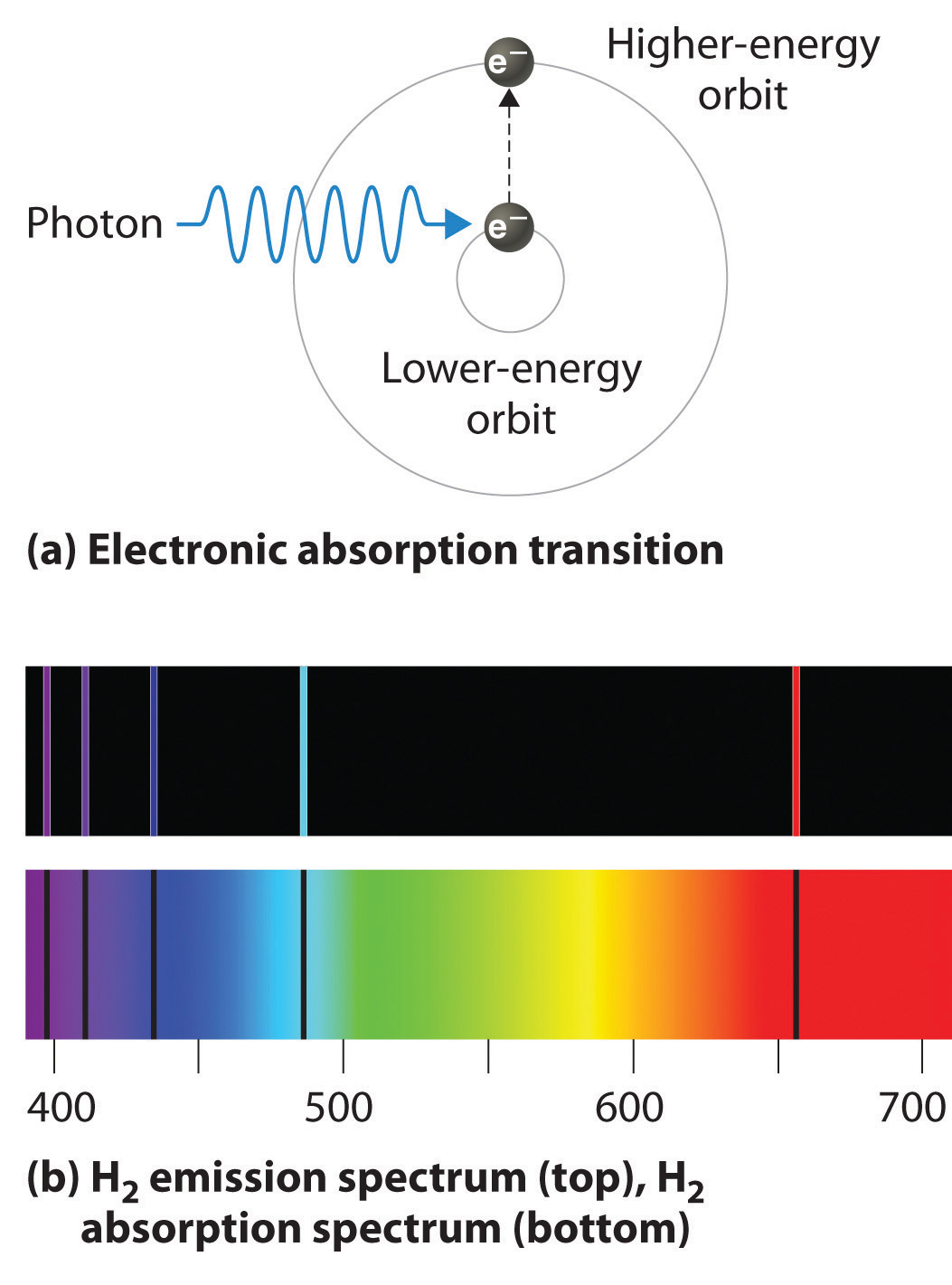
Atomic Spectra And Models Of The Atom
| Title: Atomic Spectra And Models Of The Atom |
| Format: eBook |
| Number of Pages: 273 pages What Wavelengths Appear In The Atom's Emission Spectrum |
| Publication Date: March 2017 |
| File Size: 1.5mb |
| Read Atomic Spectra And Models Of The Atom |
 |
What wavelengths are seen in the atoms emission spectrum.
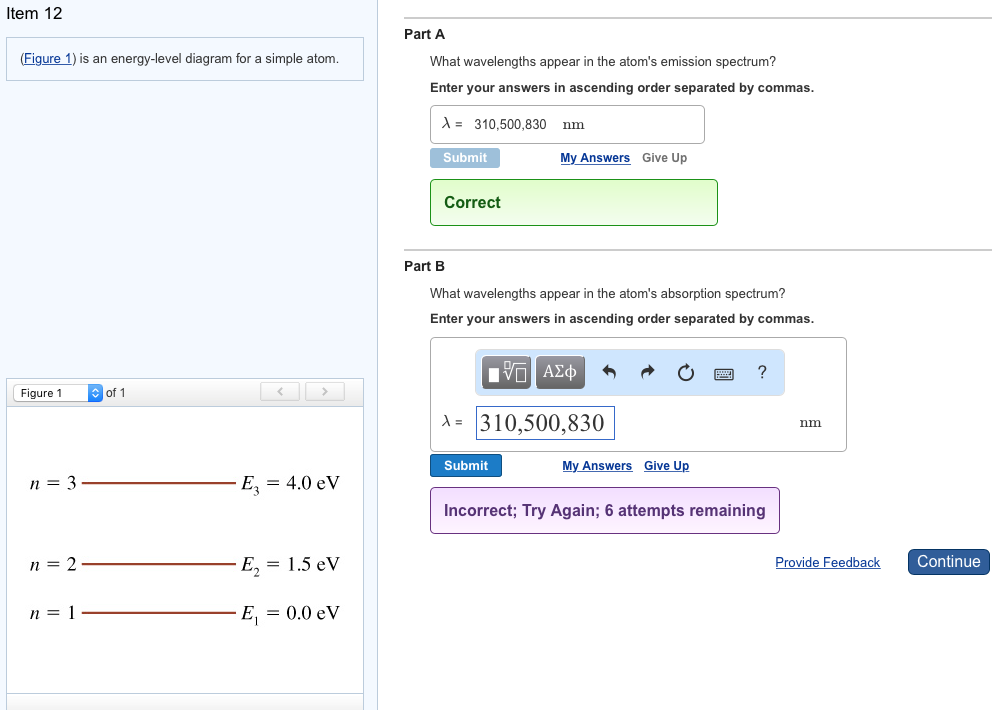
Ste Atoms transit from one state to another state. Enter your answers in ascending order separated by commas Figure 1 of 1 nm Submit Request Answer E-40 eV Provide Feedback n-2 E. 4 attempts remaining n 3 Ez 40 eV Provide Feedback Next n 2 E2 15 eV n1 E 00 eV 1 Review Constants Periodic Table An electron with 23 eV of kinetic energy collides with an atom. What wavelengths in nm appear in the atoms emission spectrum. So as it transits lets say from tree to two you will meet a four time with energy because to e tree minus 82 I think this is to conserve energy conservation off energy saying with Ah lets say from 2 to 1 you will meet energy off e tu minus you want. Enter your answers in ascending order separated by commas.

